THE NEED
A number of therapeutic options have been explored over the course of the COVID-
19 pandemic, yet there is still no effective treatment for hospitalized patients. It is
therefore crucial to identify targets for antiviral intervention and elucidate critical viral
and host determinants that play a central role in the development of severe disease.
THE SOLUTION
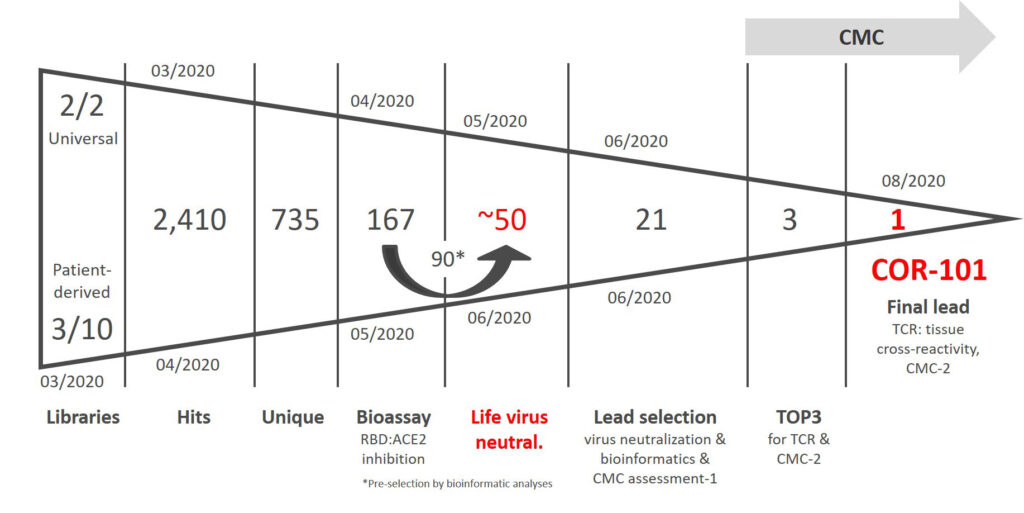
Fast track development of SARS-CoV-2 antibody (6 months)
In less than six months, an antibody gene library was generated, screened against SARS-CoV-2 S1
protein and a final lead molecule (COR-101) was identified by a set of selected assays including life
virus neutralization and CMC assessment. YUMAB‘s antibody discovery and development platform
identified several promising candidates with high affinity, very good developability prediction
and binding to most presently known SARS-CoV-2 variants. Antibodies expressed at GMP standards
after stable cell line development show a high expression titer and exhibit good developability.
THE IMPACT
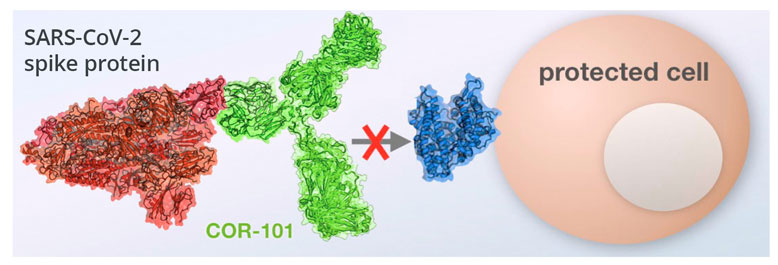
Prevention of cell infection without elevated immune response
COR-101 binds to the receptor binding domain of the spike protein of SARS-CoV-2 thereby preventing any Fc-gamma receptor binding and avoiding the risk of antibody-dependent enhancement or viral proliferation. The antibody reduces the pulmonary virus load by more than 99% three days after treatment and also binds to many mutated variants of SARS-CoV-2, such as the alpha and delta variants.
Clinical development of COR-101 lead candidate.
The lead product COR-101 has been designed for all COVID-19 patients including hospitalized individuals with moderate to severe disease.
COR-101 is currently evaluated in a Phase Ib/II clinical trial for two target main indications:
• Therapeutic treatment of infected patients to safe lives and accelerate healing
• Prophylactic treatment of high risk populations to prevent infection and onset of disease.